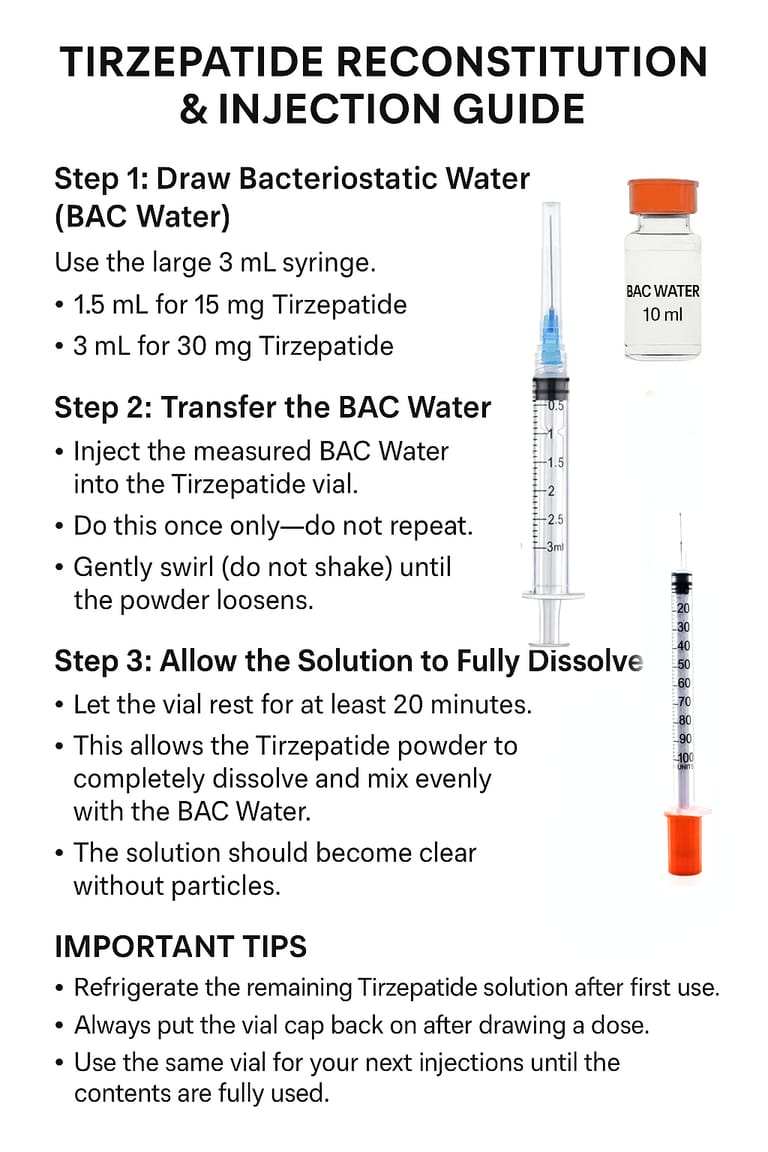
DualAgonex™ Tirzepatide
Sterile lyophilized powder for subcutaneous injection
₱21499.00₱9500.00
Out of stock
Tirzepatide is a dual-agonist GIP + GLP-1 metabolic research peptide known for strong weight-management and metabolic effects. Clinical trials show 15%–22% total body weight reduction, with improved glucose control, reduced appetite, and enhanced insulin sensitivity. Real-world users often report 18%–26% weight loss, with rare cases reaching 30%, especially at higher BMI.
Benefits: Supports appetite suppression, accelerated fat loss, improved insulin sensitivity, lower glucose levels, and better metabolic balance.
Mechanism: Dual incretin activation enhances satiety, stabilizes glucose, and increases metabolic efficiency.
Side Effects: Possible nausea, GI discomfort, diarrhea/constipation, reduced appetite. Not advised for individuals with thyroid cancer history or MEN2.
Tirzepatide (Dual Agonist Metabolic Peptide)
Tirzepatide is an advanced dual-agonist research peptide that activates both GIP and GLP-1 receptors, designed to support modern research in metabolic health, weight regulation, appetite control, and glycemic improvement. As one of the most clinically validated incretin-based peptides available, tirzepatide has earned a global reputation as an elite, high-efficacy fat-loss agent, delivering significant and sustained weight reduction in both clinical and real-world settings.
Tirzepatide’s unique dual-mechanism approach makes it significantly more potent than traditional GLP-1 monotherapies, driving faster appetite control, improved insulin function, and deep reductions in body fat.
Mechanism of Action
Tirzepatide operates using a two-hormone synergy, producing strong metabolic benefits:
1. GLP-1 Receptor Activation
Suppresses appetite and reduces cravings
Slows gastric emptying for prolonged fullness
Improves insulin release and glucose regulation
Reduces overeating and stabilizes post-meal glucose levels
2. GIP Receptor Activation
Enhances insulin secretion during meals
Improves metabolic flexibility and glucose handling
Increases energy utilization
Works synergistically with GLP-1 for amplified weight-loss effects
Together, GLP-1 + GIP create a metabolic environment that supports significant fat reduction, improved glycemic control, and more efficient long-term weight management.
Clinical Trial Findings
Clinical studies have established tirzepatide as one of the most effective metabolic compounds prior to the rise of triple-agonists like retatrutide.
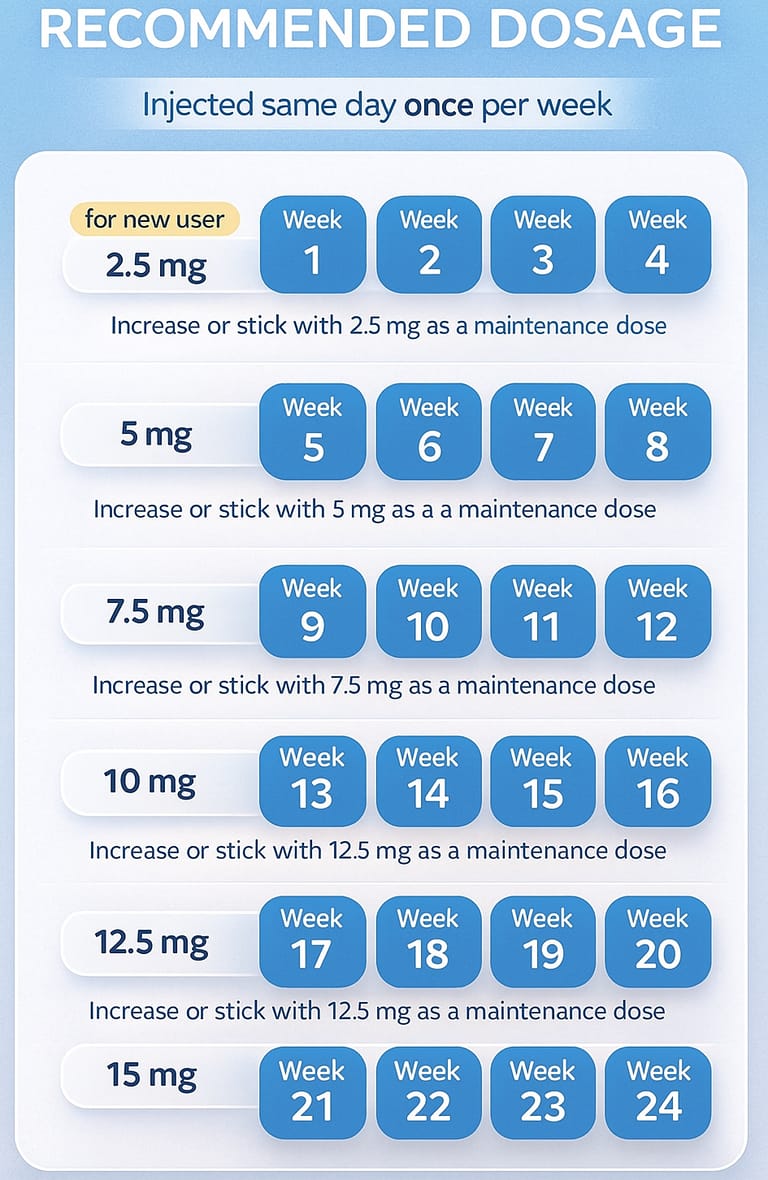
Average Weight Loss by Dose
5 mg: ~15% total body weight reduction
10 mg: ~19–20%
15 mg: 21–22.5%, the highest trial dose
Metabolic Improvements
HbA1c reduction of up to 2.0%
Reduction in triglycerides and LDL
Improved fasting glucose and insulin sensitivity
Reduction in visceral fat
These results firmly position tirzepatide as a top-tier dual agonist with substantial fat-loss potential.
Anecdotal Results (Community-Reported)
Across global user communities—including obesity forums, peptide groups, and metabolic health circles—tirzepatide reports remain consistently strong:
Faster appetite suppression compared to semaglutide
Significant energy stabilization and reduced cravings
Weight loss ranging 18%–26% for most long-term users
Exceptional outliers reaching 28%–30% (especially high-BMI individuals)
Visible reductions in belly fat (“shrinking waistline effect”)
Better mood, reduced inflammation, improved stamina and sleep
Tirzepatide is widely praised for its predictability, smooth dose progression, and strong tolerability once stabilized.
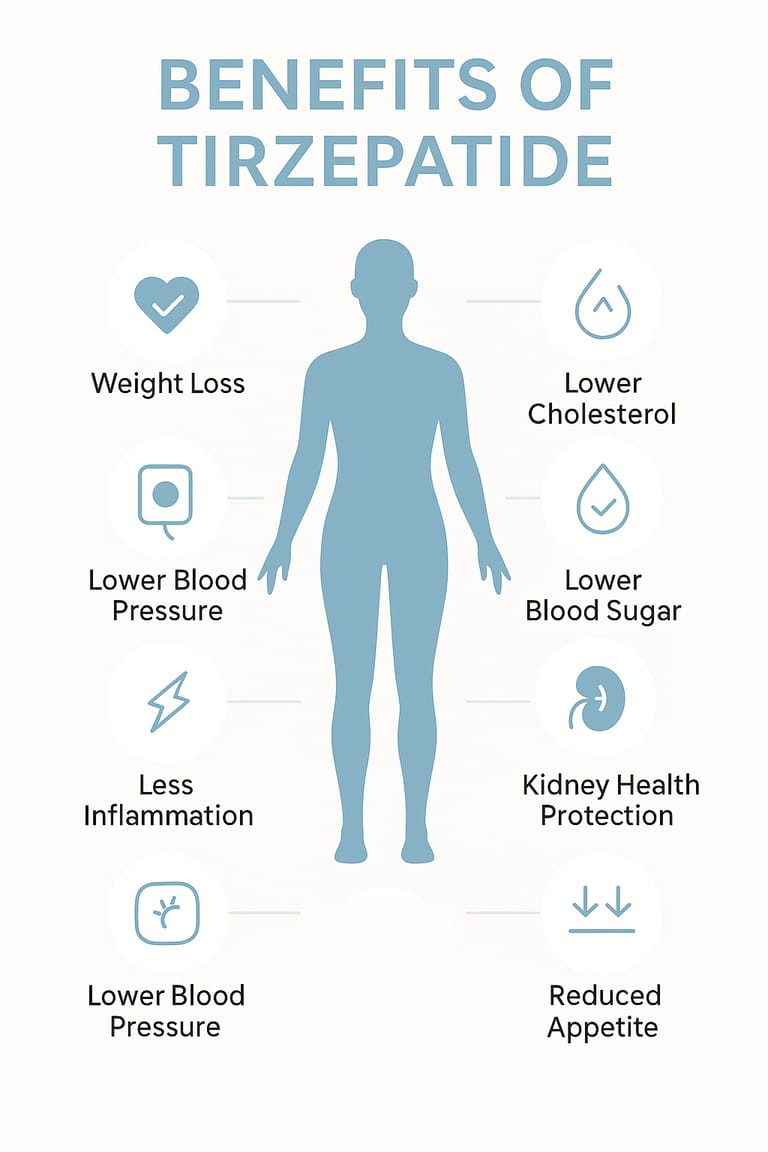
Benefits
Supports major appetite suppression
Promotes consistent and significant weight reduction
Enhances insulin sensitivity and carbohydrate metabolism
Improves cholesterol and triglyceride profiles
Supports reduction of visceral and abdominal fat
Aids research on obesity, insulin resistance, and metabolic syndrome
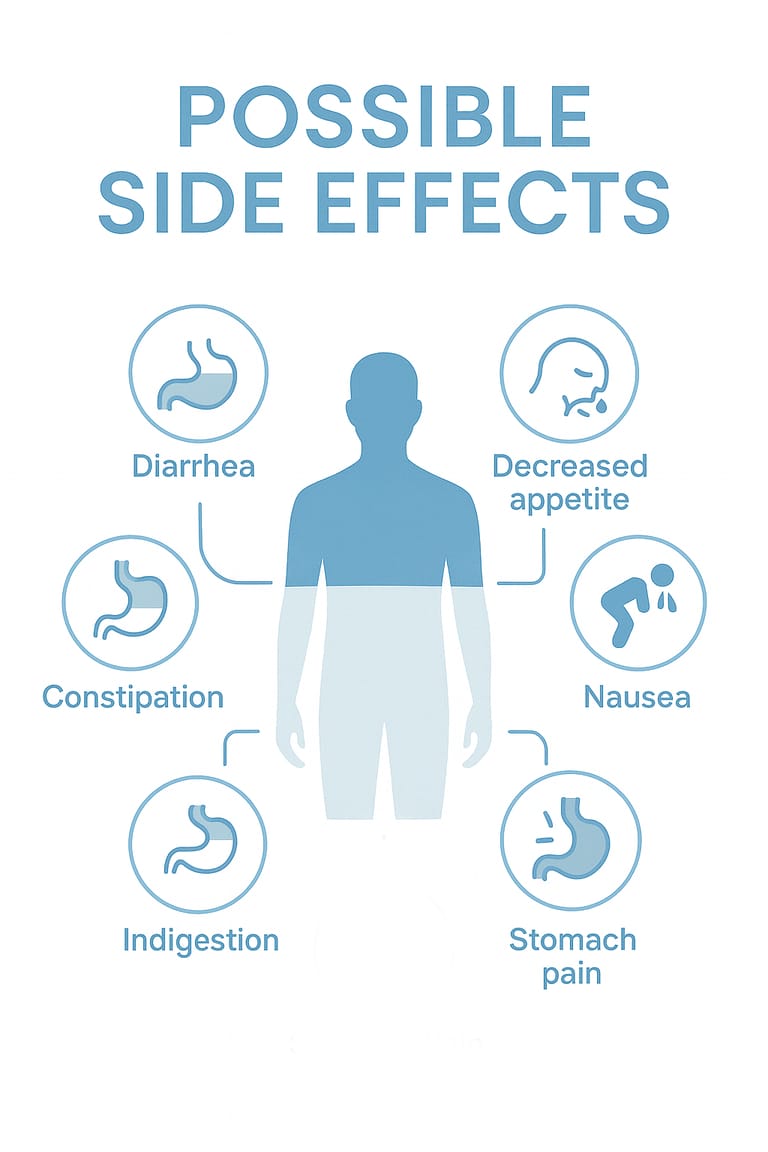
Possible Side Effects
Common dose-related adverse effects include:
Nausea
Gastrointestinal discomfort
Diarrhea or constipation
Appetite reduction
Occasional fatigue or dizziness
Contraindications:
Not advised for individuals with:
History of medullary thyroid carcinoma
MEN2 (Multiple Endocrine Neoplasia Type 2)
Why Researchers Consider Tirzepatide “S-Tier”
Delivers higher average weight-loss than semaglutide
Dual incretin approach gives superior metabolic outcomes
Strong clinical evidence base
Smooth escalation with stable long-term effects
Considered the gold standard before the arrival of retatrutide
While retatrutide may now hold the “God Mode” crown, tirzepatide remains the most proven and widely adopted incretin-based peptide in real-world practice.